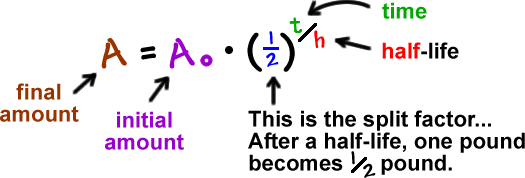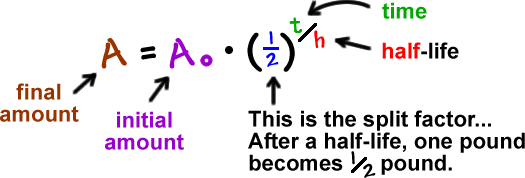
- Using Radioactive Half Life To Generate Keys Lyrics
- Radioactive Half Life Lab
Scientists look at half-life decay rates of radioactive isotopes to estimate when a particular atom might decay. A useful application of half-lives is radioactive dating. This has to do with figuring out the age of ancient things.
The half-life of a radioactive isotope refers to the amount of time required for half of a quantity of a radioactive isotope to decay. Carbon-14 has a half-life of 5730 years, which means that if you take one gram of carbon-14, half of it will decay in 5730 years. So, if radioactive iodine-131 (which has a half-life of 8 days) is injected into the body to treat thyroid cancer, it’ll be “gone” in 10 half-lives, or 80 days. Given data, generate a graph showing exponential decay. Radioactive isotopes all decay at a constant rate. While some can decay rapidly, others may decay over thousands of years. We measure the rate of decause using a unit called the half-life. The half-life of an isotope is the time it takes for one half of the substance to decay. The follow-up activity takes twenty minutes to half an hour. Overview of activity: Students generate a radioactive decay table for an imaginary element (designed to simplify the math), use their data to plot a decay graph, develop the concept of half-life, and use the graph to 'age' several samples. Aug 11, 2015 136 - Half-Life and Radioactive Decay In this video Paul Andersen explains how a radioactive nuclei can decay by releasing an alpha, beta, or gamma particle. The exact moment of decay for each. Radiometric Dating Activity Teacher Notes: This hands-on activity is a simulation of some of the radiometric dating techniques used by scientists to determine the age of a mineral or fossil. The activity uses the basic principle of radioactive half-life, and is a good follow-up lesson after the students have learned about half-life.
If you could watch a single atom of a radioactive isotope, U-238, for example, you wouldn’t be able to predict when that particular atom might decay. It might take a millisecond, or it might take a century. There’s simply no way to tell.

But if you have a large enough sample, a pattern begins to emerge. It takes a certain amount of time for half the atoms in a sample to decay. It then takes the same amount of time for half the remaining radioactive atoms to decay, and the same amount of time for half of those remaining radioactive atoms to decay, and so on. This process is shown in the following table.
Half-Life Decay of a Radioactive Isotope
| Half-Life | Percent of Radioactive Isotope Remaining |
|---|
| 0 | 100.00 |
| 1 | 50.00 |
| 2 | 25.00 |
| 3 | 12.50 |
| 4 | 6.25 |
| 5 | 3.12 |
| 6 | 1.56 |
| 7 | 0.78 |
| 8 | 0.39 |
| 9 | 0.19 |
| 10 | 0.09 |
The amount of time it takes for one-half of a sample to decay is called the half-life of the isotope, and it’s given the symbol:
It’s important to realize that the half-life decay of radioactive isotopes is not linear. For example, you can’t find the remaining amount of an isotope as 7.5 half-lives by finding the midpoint between 7 and 8 half-lives. This decay is an example of an exponential decay, shown in the figure below.
Safe handling of radioactive material
Knowing about half-lives is important because it enables you to determine when a sample of radioactive material is safe to handle. The rule is that a sample is safe when its radioactivity has dropped below detection limits. And that occurs at 10 half-lives.
Generate ssh keys linux rsa. So, if radioactive iodine-131 (which has a half-life of 8 days) is injected into the body to treat thyroid cancer, it’ll be “gone” in 10 half-lives, or 80 days.
This stuff is important to know when using radioactive isotopes as medical tracers, which are taken into the body to allow doctors to trace a pathway or find a blockage, or in cancer treatments. They need to be active long enough to treat the condition, but they should also have a short enough half-life so that they don’t injure healthy cells and organs.
Radioactive dating
Radioactive dating is helpful for figuring out the age of ancient things. Carbon-14 (C-14), a radioactive isotope of carbon, is produced in the upper atmosphere by cosmic radiation. The primary carbon-containing compound in the atmosphere is carbon dioxide, and a very small amount of carbon dioxide contains C-14.
Plants absorb C-14 during photosynthesis, so C-14 is incorporated into the cellular structure of plants. Plants are then eaten by animals, making C-14 a part of the cellular structure of all living things.
Using Radioactive Half Life To Generate Keys Lyrics
As long as an organism is alive, the amount of C-14 in its cellular structure remains constant. But when the organism dies, the amount of C-14 begins to decrease. Scientists know the half-life of C-14 (5,730 years), so they can figure out how long ago the organism died.
Radioactive Half Life Lab
Carbon-14 dating can only be used to determine the age of something that was once alive. It can’t be used to determine the age of a moon rock or a meteorite. For nonliving substances, scientists use other isotopes, such as potassium-40.