Published online 2018 Nov 7.
- Next Generation Sequencing Overview
- Next Generation Sequencing Basics
- Dec 08, 2009 The major advance offered by next-generation sequencing (NGS) technologies is the ability to produce, in some cases, in excess of one billion.
- Elbaidouri M., Chaparro C., Panaud O. (2013) Use of Next Generation Sequencing (NGS) Technologies for the Genome-Wide Detection of Transposition. In: Peterson T. (eds) Plant Transposable Elements. Methods in Molecular Biology (Methods and Protocols), vol 1057. Humana Press, Totowa, NJ. First Online 05 July 2013.
- Nov 14, 2016 Sample to Insight 45 Overview of NGS technologies and innovative NGS library prep methods Part 1: Introduction to next-generation sequencing (NGS) technology Part 2: Innovative NGS library construction technology Part 3: Advanced NGS library prep for challenging samples Upcoming webinars Intro to NGS, 46.
- Apr 30, 2018 The next-generation sequencing (NGS) has been introduced in genomic laboratories about 10 years ago. Its impact on technological revolution has important implications in human biology and medicine. After improvements in accuracy, robustness and handling, it became a widely used and an alternative approach to the direct Sanger sequencing 2,3.
- Sequencing key words Next Generation Sequencing (NGS) or High-throughput Sequencing (HTS) are catch-all terms that describe the modern sequencing technologies. By sequencing the DNA (or RNA) faster than previous technologies, NGS are conquering the research in biology providing a quick access to biological sequences, genes expression level.
PMID: 30479607
This article has been cited by other articles in PMC.
Abstract
Translating the power of high-throughput sequencing technologies from research area into clinical medicine is one of the major goal for several researchers and health-care providers. One of the important advantages of these technologies is that they can be successfully used in a numerous range of clinical applications. The efficiency of sequencing, that can now be achieved, is leading impressive progress in the diagnostics of common and rare genetic disorders, inherited forms of cancer, prenatal testing or infectious diseases, to cite some examples. Despite several challenges and limitations still remain to overcome, the high-throughput sequencing technologies are leading to real and unprecedented benefits for the medical care of patients.
Key words: next generation sequencing, clinical laboratory medicine, medical care, high-throughput approach
GENERAL OVERVIEW
Over the past decade great advances have been done in sequencing technologies. After Sanger Sequencing, the current gold standard approach, also known as dideoxy method [], high-throughput sequencing has been developed and widespread in biomedical laboratories. The first one allows to analyse one DNA segment at time in laborious and time-consuming way while the second approach has the great advantage of performing a simultaneous analysis of several genomic regions, with a dramatic reduction also of the cost of sequencing per base [].
Sanger sequencing vs Next generation sequencing? (Illumina in this example but the concept is the same for other NGS technologies) you prepare a target-DNA library and you load it into a.
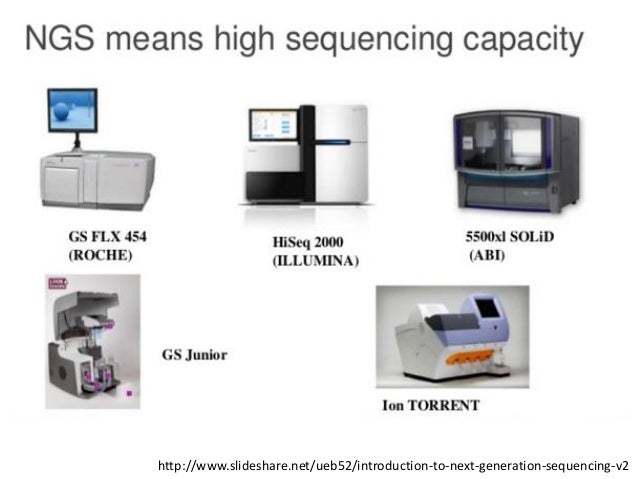
Today the high-throughput next generation sequencing (NGS) instruments mainly used in biomedical laboratories are the Ion Torrent sequencers (Life Technologies) and the Illumina platforms (Illumina) [,].
All NGS technologies are based on the same general process, comprising template preparation, sequencing and data analysis. The unique combination of specific technical details distinguishes one technology from another and determines the type of data produced from each platform [].
After the extraction of DNA, the first step of the sequencing process is the library preparation, which consists on the ligation of DNA fragments to platform specific oligonucleotide adapters []. After that each fragment is immobilized and clonally amplified. In Life Technologies approach, clonal amplification is performed by emulsion PCR, in which DNA fragments are amplified on the beads surface in oil-aqueous mixture []. Illumina approach otherwise is based on a unique “isothermal bridge amplification” reaction that occurs on the surface of the flow cell [].
For sequencing Life Technologies exploited the native dNTP chemistry during base incorporation by DNA polymerase, that relies hydrogen ions, causing the pH modification that is detected by a modified silicon chip []. Illumina sequencing is instead performed on a flow-cell and it is based on the existing Solexa sequencing by synthesis chemistry, based on the fluorescent detection released when the complementary fluorescently tagged nucleotides are incorporated [].
In recent years, the advent of these benchtop NGS platforms on the marketplace has had an impressive impact in -omics, thanks to the huge amount of data obtained with a significant reduction of time and costs []. Indeed NGS has been applied in varied contexts, restricted not only to genomics but also to transcriptomics and epigenomics, such as in non-coding RNA expression profiling, finding transcription factor binding sites, RNA seq, ChIp-Seq or MeDIP, to cite few examples [].
In research genetic studies NGS has been successfully exploited to identify new causative genes or variants associated to inherited diseases, especially in genetically heterogenous disorders, whose genetic basis was partially unknown and in which gene-by-gene Sanger sequencing approach would not have been economical or efficient []. For this purpose, NGS has been applied to whole-genome, exome or targeted sequencing, leading to the improvement of the current knowledge of genetic basis of several pathologies, such as retinitis pigmentosa, cardiomyopathies or inherited cancer [].
More recently the widespread use of these rapid high-throughput technologies, the improvement of their performance and the overcoming of initial technical limitations are encouraging their transition from basic research into clinics with important benefits for routine patient management.
USE OF NGS IN THE CLINICAL PRACTICE
Now NGS is an established test method in many clinical laboratories, in particular for the detection of germline and somatic genetic mutations. The analysis of causative mutations in inherited diseases is performed using different approaches, exploiting targeted panel, whole exome, whole genome or mitochondrial DNA sequencing [,]. More in details, targeted panel analysis is usually applied to genetic test for different genetically heterogeneous disorders, such as renal, neurologic, connective tissue disorders, cardiomyopathies, immune deficiencies, blindness, deafness, and several forms of inherited cancer [,].
Even if the analyzed gene panel may vary between laboratories, target sequencing is the first approach of genetic test for inherited disorders, while whole exome sequencing is exploited for negative cases, in which targeted testing has not been informative. Moreover, whole exome approach is useful in rare diseases for trio testing, sequencing the child and both parents [].
In oncology, targeted testing is widely used, exploiting two different approaches. In the first one the targeted panel may be focused only on principle genes associated to a particular type of malignancy, for example BRCA1 and BRCA2 gene for breast and ovarian cancer, while in the other one NGS approach allows to analyze a broader panel including genes associated with other cancers. Given the clinical overlapping between different forms of cancer, for example between ovarian cancer and Lynch syndrome, this latter approach may be useful to enhance the diagnostic yield []. Battlefield 5 cd key generator. In oncology, whole exome and whole genome sequencing are not currently used for clinical purpose, in order to avoid the potential risk of unactionable incidental findings [,].
More recently, several new NGS applications moved to the research area to clinical use, citing for example the analysis of cell-free DNA in the prenatal genetic-testing [], circulating tumor DNA testing [,32], human leukocyte antigen (HLA) typing [], microbial analysis [], RNA sequencing and expression [], and methylation[], even if there are yet some challenges to overcome.
For example in HLA typing, it is difficult to differentiate low-frequency alleles from high-frequency artifacts and newer data analysis approach or the development of instruments for single-molecule sequencing, called third-generation sequencers, are solving these limitations [,].
Today testing of circulating tumor DNA (ctDNA), often referred to as “liquid biopsy”, is now available in clinics [,]. One possible approach is NGS, that presents a lot of potential applications, including diagnosis of cancer, monitoring for progression or relapse, and targeted therapy for a patient with a known cancer diagnosis [32,]. Indeed, several studies have shown that ctDNA sequencing allows at first to detect somatic mutation in patients with known cancer diagnosis and then to monitor it in correlation with the relapse and progression of disease []. Without doubt the detection of ctDNA using NGS presents the great advantage to be a reasonable alternative to the repeated invasive biopsies for patients with metastatic cancers. However, it still presents some limitations due to a low sensitivity to detect early-stage cancer (false negatives), limiting until now the practical use of ctDNA for early cancer diagnosis or screening [].
Other clinical applications of NGS include pharmacogenetics and microbial sequencing but these topics are beyond the scope of this article.
Although NGS is now widely used in clinics, several challenges still remain to overcome.
The main issues are, for example, the sequencing of genomic portions that are difficult to analyze, due their intrinsic features (pseudogenes, homologous regions, repetitive regions, GC-rich regions) and the limited ability to detect structural gene variation and copy number variation (CNV) []. Sometimes the storage and the interpretation of huge amounts of sequence data, mainly of several novel or rare mutations, by trained health care professionals may be still an open challenge [,]. Moreover, a successful NGS testing need a collaborative effort between geneticists and physicians to combine and integrate clinical data and genetic analyses to guide medical care of patient.
CONCLUSIONS
NGS technologies have revolutionized biological research and have deeply transform the fields of diagnostic pathology and clinical medicine. In the future, the use of NGS in clinical laboratories will surely increase as technology and bioinformatics, in order to address the current limitations, improve the quality of results, and increase the number of possible clinical applications.
However, the challenge for clinical laboratories will be to perform the most appropriate approach of NGS testing taking into account the clinical relevance, cost-effectiveness and clinical care of patient.
REFERENCES
1. Sanger F, Nicklen S, Coulson AR.DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74: 5463-5467. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=431765&tool=pmcentrez&rendertype=abstract[PMC free article] [PubMed] [Google Scholar]
2. Jamuar SS, Tan E-C.Clinical application of next-generation sequencing for Mendelian diseases. Hum Genomics. 2015;9: 10doi:10.1186/s40246-015-0031-5 [PMC free article] [PubMed] [Google Scholar]
3. Di Resta C, Galbiati S, Carrera P, Ferrari M.Next-generation sequencing approach for the diagnosis of human diseases: open challenges and new opportunities. EJIFCC. 2018;29: 4-14. Available: http://www.ncbi.nlm.nih.gov/pubmed/29765282[PMC free article] [PubMed] [Google Scholar]
4. Mardis ER.Next-generation sequencing platforms. Annu Rev Anal Chem (Palo Alto Calif). 2013;6: 287–303. doi:10.1146/annurev-anchem-062012-092628 [PubMed] [Google Scholar]
5. Reuter JA, Spacek D V, Snyder MP.High-throughput sequencing technologies. Mol Cell. 2015;58: 586–597. doi:10.1016/j.molcel.2015.05.004 [PMC free article] [PubMed] [Google Scholar]
6. McKernan KJ, Peckham HE, Costa GL, McLaughlin SF, Fu Y, Tsung EF, et al. Sequence and structural variation in a human genome uncovered by short-read, massively parallel ligation sequencing using two-base encoding. Genome Res. 2009;19: 1527–1541. doi:10.1101/gr.091868.109 [PMC free article] [PubMed] [Google Scholar]
7. Wheeler DA, Srinivasan M, Egholm M, Shen Y, Chen L, McGuire A, et al. The complete genome of an individual by massively parallel DNA sequencing. Nature. 2008;452:872–876. doi:10.1038/nature06884 [PubMed] [Google Scholar]
8. Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, Bemben LA, et al. Genome sequencing in microfabricated high-density picolitre reactors. Nature. 2005;437:376–380. doi:10.1038/nature03959 [PMC free article] [PubMed] [Google Scholar]
9. Bentley DR, Balasubramanian S, Swerdlow HP, Smith GP, Milton J, Brown CG, et al. Accurate whole human genome sequencing using reversible terminator chemistry. Nature. 2008;456: 53–59. doi:10.1038/nature07517 [PMC free article] [PubMed] [Google Scholar]
10. Merriman B, Rothberg JM.Progress in ion torrent semiconductor chip based sequencing. Electrophoresis. 2012;33: 3397–3417. doi:10.1002/elps.201200424 [PubMed] [Google Scholar]
11. Quail M, Smith ME, Coupland P, Otto TD, Harris SR, Connor TR, et al. A tale of three next generation sequencing platforms: comparison of Ion torrent, pacific biosciences and illumina MiSeq sequencers. BMC Genomics. BioMed Central; 2012;13: 341doi:10.1186/1471-2164-13-341 [PMC free article] [PubMed] [Google Scholar]
12. Williams ES, Hegde M.Implementing genomic medicine in pathology. Adv Anat Pathol. 2013;20: 238–244. doi:10.1097/PAP.0b013e3182977199 [PubMed] [Google Scholar]
13. Choi BY, Kim BJ.Application of next generation sequencing upon the molecular genetic diagnosis of deafness. Korean J Audiol. Korean Audiological Society; 2012;16: 1–5. doi:10.7874/kja.2012.16.1.1 [PMC free article] [PubMed] [Google Scholar]
14. Carrera P, Di Resta C, Volonteri C, Castiglioni E, Bonfiglio S, Lazarevic D, et al. Exome sequencing and pathway analysis for identification of genetic variability relevant for bronchopulmonary dysplasia (BPD) in preterm newborns: A pilot study. Clin Chim Acta. 2015;451: 39–45. doi:10.1016/j.cca.2015.01.001 [PubMed] [Google Scholar]
15. Di Resta C, Spiga I, Presi S, Merella S, Pipitone GB, Manitto MP, et al. Integration of multigene panels for the diagnosis of hereditary retinal disorders using Next Generation Sequencing and bioinformatics approaches. EJIFCC. 2018;29: 15–25. Available: http://www.ncbi.nlm.nih.gov/pubmed/29765283[PMC free article] [PubMed] [Google Scholar]
16. Doyle MA, Li J, Doig K, Fellowes A, Wong SQ.Studying Cancer Genomics Through Next-Generation DNA Sequencing and Bioinformatics. Methods in molecular biology (Clifton, NJ). 2014. pp. 83–98. doi:10.1007/978-1-4939-0847-9_6 [PubMed] [Google Scholar]
17. Di Resta C, Pietrelli A, Sala S, Della Bella P, De Bellis G, Ferrari M, et al. High-throughput genetic characterization of a cohort of Brugada syndrome patients. Hum Mol Genet. 2015;24: 5828–5835. doi:10.1093/hmg/ddv302 [PubMed] [Google Scholar]
18. Strom SP.Current practices and guidelines for clinical next-generation sequencing oncology testing. Cancer Biol Med. Chinese Anti-Cancer Association; 2016;13: 3–11. doi:10.28092/j.issn.2095-3941.2016.0004 [PMC free article] [PubMed] [Google Scholar]
19. Yohe S, Thyagarajan B.Review of Clinical Next-Generation Sequencing. Arch Pathol Lab Med. 2017;141: 15441557doi:10.5858/arpa.2016-0501-RA [PubMed] [Google Scholar]
20. Kamps R, Brandão RD, van den Bosch BJ, Paulussen ADC, Xanthoulea S, Blok MJ, et al. Next-Generation Sequencing in Oncology: Genetic Diagnosis, Risk Prediction and Cancer Classification. Int J Mol Sci. Multidisciplinary Digital Publishing Institute (MDPI); 2017;18doi:10.3390/ijms18020308 [PMC free article] [PubMed] [Google Scholar]
21. Celestino-Soper PBS, Gao H, Lynnes TC, Lin H, Liu Y, Spoonamore KG, et al. Validation and Utilization of a Clinical Next-Generation Sequencing Panel for Selected Cardiovascular Disorders. Front Cardiovasc Med. 2017;4:11doi:10.3389/fcvm.2017.00011 [PMC free article] [PubMed] [Google Scholar]
22. Weerakkody RA, Vandrovcova J, Kanonidou C, Mueller M, Gampawar P, Ibrahim Y, et al. Targeted next-generation sequencing makes new molecular diagnoses and expands genotype-phenotype relationship in Ehlers-Danlos syndrome. Genet Med. 2016;18: 1119–1127. doi:10.1038/gim.2016.14 [PubMed] [Google Scholar]
23. Nijman IJ, van Montfrans JM, Hoogstraat M, Boes ML, van de Corput L, Renner ED, et al. Targeted next-generation sequencing: A novel diagnostic tool for primary immunodeficiencies. J Allergy Clin Immunol. 2014;133:529–534.e1. doi:10.1016/j.jaci.2013.08.032 [PubMed] [Google Scholar]
24. Fernandez-Marmiesse A, Gouveia S, Couce ML.NGS Technologies as a Turning Point in Rare Disease Research, Diagnosis and Treatment. Curr Med Chem. 2018;25: 404–432. doi:10.2174/0929867324666170718101946 [PMC free article] [PubMed] [Google Scholar]
25. Yang Y, Muzny DM, Reid JG, Bainbridge MN, Willis A, Ward PA, et al. Clinical Whole-Exome Sequencing for the Diagnosis of Mendelian Disorders. N Engl J Med. 2013;369: 1502–1511. doi:10.1056/NEJMoa1306555 [PMC free article] [PubMed] [Google Scholar]
26. Sawyer SL, Hartley T, Dyment DA, Beaulieu CL, Schwartzentruber J, Smith A, et al. Utility of whole-exome sequencing for those near the end of the diagnostic odyssey: time to address gaps in care. Clin Genet. 2016;89: 275–284. doi:10.1111/cge.12654 [PMC free article] [PubMed] [Google Scholar]
27. Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW, et al. ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. NIH Public Access; 2015;110: 223–262; quiz 263. doi:10.1038/ajg.2014.435 [PMC free article] [PubMed] [Google Scholar]
28. Park HS, Park S-J, Kim JY, Kim S, Ryu J, Sohn J, et al. Next-generation sequencing of BRCA1/2 in breast cancer patients: potential effects on clinical decision-making using rapid, high-accuracy genetic results. Ann Surg Treat Res. Korean Surgical Society; 2017;92: 331–339. doi:10.4174/astr.2017.92.5.331 [PMC free article] [PubMed] [Google Scholar]
29. O’Leary E, Iacoboni D, Holle J, Michalski ST, Esplin ED, Yang S, et al. Expanded Gene Panel Use for Women With Breast Cancer: Identification and Intervention Beyond Breast Cancer Risk. Ann Surg Oncol. Springer; 2017;24:3060–3066. doi:10.1245/s10434-017-5963-7 [PMC free article] [PubMed] [Google Scholar]
30. Ordulu Z, Kammin T, Brand H, Pillalamarri V, Redin CE, Collins RL, et al. Structural Chromosomal Rearrangements Require Nucleotide-Level Resolution: Lessons from Next-Generation Sequencing in Prenatal Diagnosis. Am J Hum Genet. Elsevier; 2016;99: 1015–1033. doi:10.1016/j.ajhg.2016.08.022 [PMC free article] [PubMed] [Google Scholar]
31. Thompson JC, Yee SS, Troxel AB, Savitch SL, Fan R, Balli D, et al. Detection of Therapeutically Targetable Driver and Resistance Mutations in Lung Cancer Patients by Next-Generation Sequencing of Cell-Free Circulating Tumor DNA. Clin Cancer Res. 2016;22: 5772–5782. doi:10.1158/1078-0432.CCR-16-1231 [PMC free article] [PubMed] [Google Scholar]
32. Vendrell JA, Mau-Them FT, Béganton B, Godreuil S, Coopman P, Solassol J.Circulating Cell Free Tumor DNA Detection as a Routine Tool forLung Cancer Patient Management. Int J Mol Sci. Multidisciplinary Digital Publishing Institute (MDPI); 2017;18doi:10.3390/ijms18020264 [Google Scholar]
33. Weimer ET, Montgomery M, Petraroia R, Crawford J, Schmitz JL.Performance Characteristics and Validation of Next-Generation Sequencing for Human Leucocyte Antigen Typing. J Mol Diagnostics. 2016;18: 668–675. doi:10.1016/j.jmoldx.2016.03.009 [PubMed] [Google Scholar]
34. Szolek A, Schubert B, Mohr C, Sturm M, Feldhahn M, Kohlbacher O.OptiType: precision HLA typing from next-generation sequencing data. Bioinformatics. 2014;30: 3310–3316. doi:10.1093/bioinformatics/btu548 [PMC free article] [PubMed] [Google Scholar]
35. Brambati C, Galbiati S, Xue E, Toffalori C, Crucitti L, Greco R, et al. Droplet digital polymerase chain reaction for DNMT3A and IDH1/2 mutations to improve early detection of acute myeloid leukemia relapse after allogeneic hematopoietic stem cell transplantation. Haematologica. 2016;101: e157–e161. doi:10.3324/haematol.2015.135467 [PMC free article] [PubMed] [Google Scholar]
36. Whale AS, Devonshire AS, Karlin-Neumann G, Regan J, Javier L, Cowen S, et al. International Interlaboratory Digital PCR Study Demonstrating High Reproducibility for the Measurement of a Rare Sequence Variant. Anal Chem. 2017;89: 1724–1733. doi:10.1021/acs.analchem.6b03980 [PubMed] [Google Scholar]
37. Ai B, Liu H, Huang Y, Peng P.Circulating cell-free DNA as a prognostic and predictive biomarker in non-small cell lung cancer. Oncotarget. 2016;7: 44583–44595. doi:10.18632/oncotarget.10069 [PMC free article] [PubMed] [Google Scholar]
38. Hofman P.Liquid biopsy for early detection of lung cancer. Curr Opin Oncol. 2017;29: 73–78. doi:10.1097/CCO.0000000000000343 [PubMed] [Google Scholar]
39. Yamada T, Iwai T, Takahashi G, Kan H, Koizumi M, Matsuda A, et al. Utility of KRAS mutation detection using circulating cell-free DNA from patients with colorectal cancer. Cancer Sci. 2016;107: 936–943. doi:10.1111/cas.12959 [PMC free article] [PubMed] [Google Scholar]
40. Wall JD, Tang LF, Zerbe B, Kvale MN, Kwok P-Y, Schaefer C, et al. Estimating genotype error rates from high-coverage next-generation sequence data. Genome Res. 2014;24: 1734–1739. doi:10.1101/gr.168393.113 [PMC free article] [PubMed] [Google Scholar]
41. Yamamoto T, Shimojima K, Ondo Y, Imai K, Chong PF, Kira R, et al. Challenges in detecting genomic copy number aberrations using next-generation sequencing data and the eXome Hidden Markov Model: a clinical exomefirst diagnostic approach. Hum genome Var. 2016;3: 16025doi:10.1038/hgv.2016.25 [PMC free article] [PubMed] [Google Scholar]
42. Li J, Dai H, Feng Y, Tang J, Chen S, Tian X, et al. A Comprehensive Strategy for Accurate Mutation Detection of the Highly Homologous PMS2. J Mol Diagn. 2015;17:545–553. doi:10.1016/j.jmoldx.2015.04.001 [PubMed] [Google Scholar]
43. Zhang T-H, Wu NC, Sun R.A benchmark study on error-correction by read-pairing and tag-clustering in amplicon-based deep sequencing. BMC Genomics. 2016;17:108doi:10.1186/s12864-016-2388-9 [PMC free article] [PubMed] [Google Scholar]
Next Generation Sequencing Overview
44. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17: 405-424. doi:10.1038/gim.2015.30 [PMC free article] [PubMed] [Google Scholar]
45. Amendola LM, Jarvik GP, Leo MC, McLaughlin HM, Akkari Y, Amaral MD, et al. Performance of ACMG-AMP Variant-Interpretation Guidelines among Nine Laboratories in the Clinical Sequencing Exploratory Research Consortium. Am J Hum Genet. 2016;98: 1067–1076. doi:10.1016/j.ajhg.2016.03.024 [PMC free article] [PubMed] [Google Scholar]
Next Generation Sequencing Basics
Articles from EJIFCC are provided here courtesy of International Federation of Clinical Chemistry and Laboratory Medicine
